Twelve years ago I started an ambitious project, a series of webpages to explain that we can be considered to be Star Children, in the sense that all the atoms forming our body (with the exception of hydrogen), have been formed in the interior of stars.
The project was too ambitious, here is a screenshot of he small part I managed to complete. It is still available on my website. Click on the screenshot to have a look.

An adult human body (70 kg) contains roughly 7×1027 atoms. Written out a 7 with 27 zeros: 7,000,000,000,000,000,000,000,000,000. That is a lot, actually much more than the estimated number of stars in the observable universe (1023-1024).
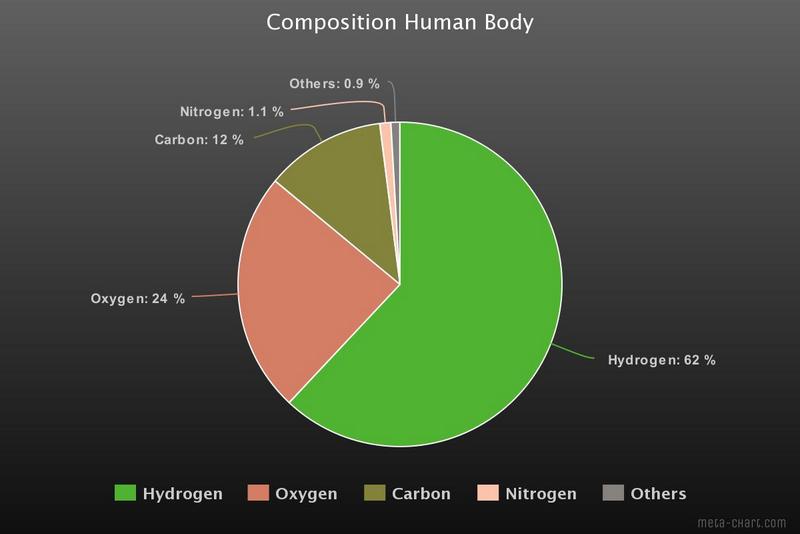
Most of those atoms are hydrogen atoms. The human body consists roughly of 60% water and each water molecule contains 2 hydrogen atoms and 1 oxygen atom. Oxygen comes second and carbon third. Together with nitrogen these four elements form more than 99% of the human body. For the graphs I have used data from the Wikipedia article Composition of the Human Body. Most of the graphs you find on the Internet give mass percentages, in that case oxygen takes first place. I prefer a representation in atomic percentages.
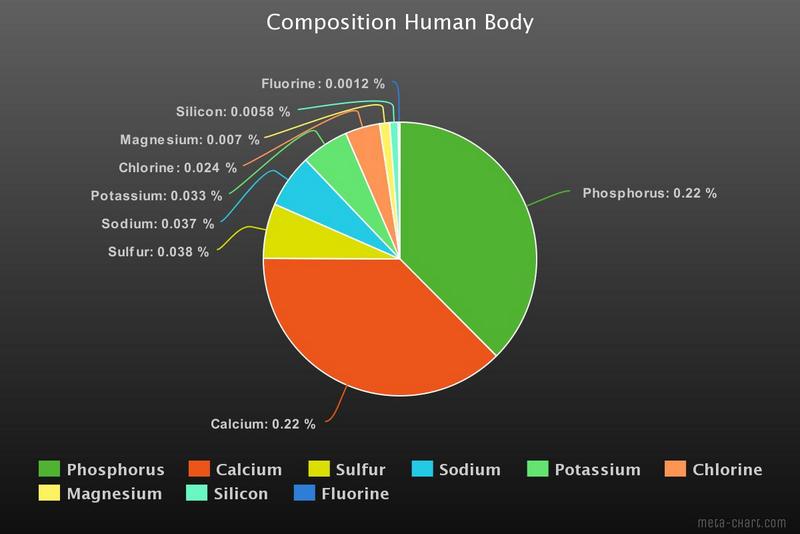
In this graph some of the other elements are shown. About 25 elements are considered to be essential for human life, some in minuscule percentages. For example Cobalt (in vitamin B12) contributes only 3.0×10−7 %
How, where and when were all these elements formed? The scientific name for the formation of the various elements is called nucleosynthesis.
Let’s start with the beginning, the Big Bang.
About 13.77 billion year ago our Universe came into being. That is the present estimate, with an uncertainty of ± 40 million year. It was unbelievably dense and hot, a “soup” of quarks gluons and photons. Immediately it started expanding and cooling and after a few minutes protons and neutrons could form. Some of these protons and neutrons fused into alpha particles ( two protons and two neutrons) until after about 20 minutes the temperature was too low for fusion.
But still way too hot for (neutral) atoms to form, it was a plasma (protons, alpha particles and electrons). If an electron and a nucleus would combine, the photons would immediately break it up again. Only after ~380.000 year, when the temperature had dropped to ~ 3000 K, electrons could recombine with protons and alpha particles to form H and He atoms. Roughly about 92% hydrogen atoms and 8% helium atoms. (usually mass percentages are given, 25% He and 75% H).
From that time onwards the photons did not interact anymore with matter, the universe was still bathing in an orange glow (3000K) but as the universe kept expanding and cooling, this radiation went from visible light to infrared , microwave etc. It is what we now still detect as the cosmic background radiation at a temperature of 2.275 K. About 1 million year after the Big Bang, the universe was COMPLETELY DARK!
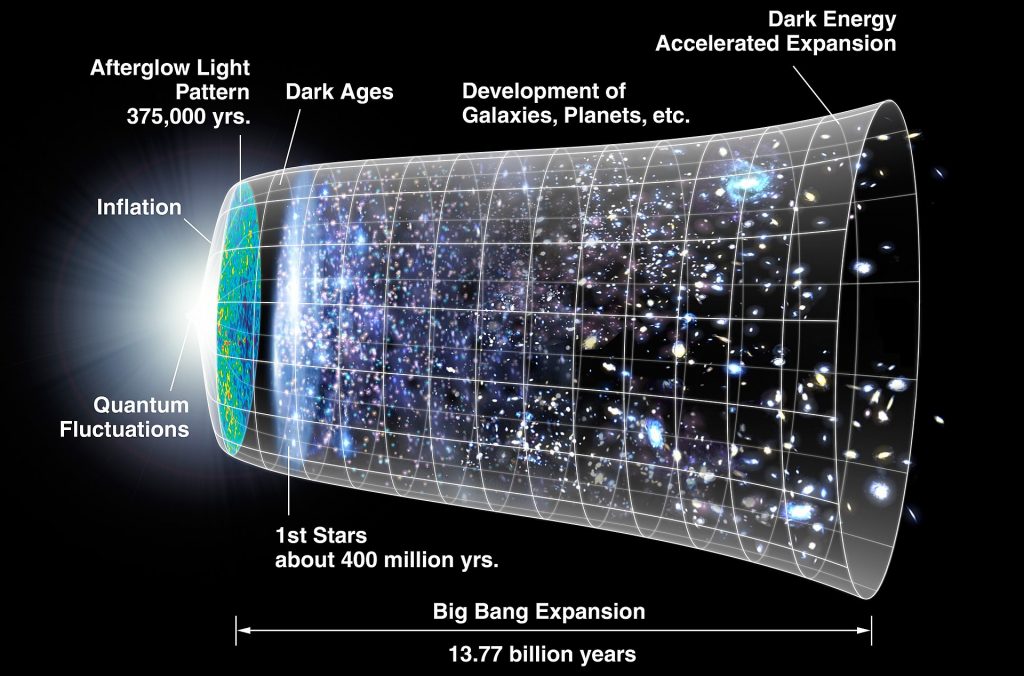
These cosmic Dark Ages lasted for many million years. But small fluctuations in matter density caused gravity to form concentrations of matter. Inside these matter concentrations the temperature was rising until a level (many millions of degrees) that fission became possible again. The first stars were born and there was again light in the universe. Later also galaxies developed and after about 1 billion years the universe was basically like it is now.
Here is an impression of the development of the universe.
We will now concentrate on the evolution of these stars. First a few general remarks. Basically everything that happens in the universe is the result of four fundamental forces.
- The strong nuclear force between nucleons, only active when the nucleons are very close together,”short-range”
- The electromagnetic force, ~100 times weaker, but “long range”, holds atoms together.
- The weak nuclear force, ~ 1 million times weaker, “short-range”, responsible for radioactivity.
- The gravitational force, extremely weak, ~1039 times weaker, “long-range”.
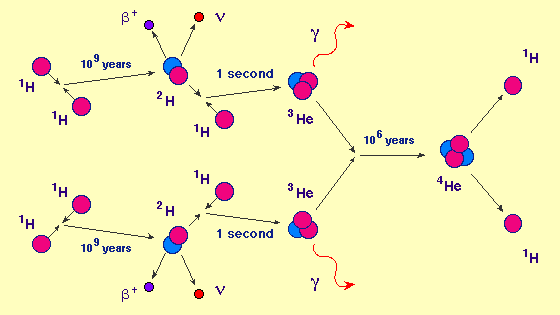
Back to the new-born star. All four forces are active here. The gravitational force tries to contract the star further. The strong force generates counter pressure, by fusing nucleons together, but those nucleons need to move fast (= high temperature) to overcome the electromagnetic repulsion. The weak nuclear force is needed to transform protons into neutrons. Here is how four protons can produce an alpha particle. Other options are also possible.
Our Sun was born 4.6 billion years ago as a relatively small star, a yellow dwarf. At the moment it is still “burning” hydrogen in its core and will continue to do that for another 5 billion years.
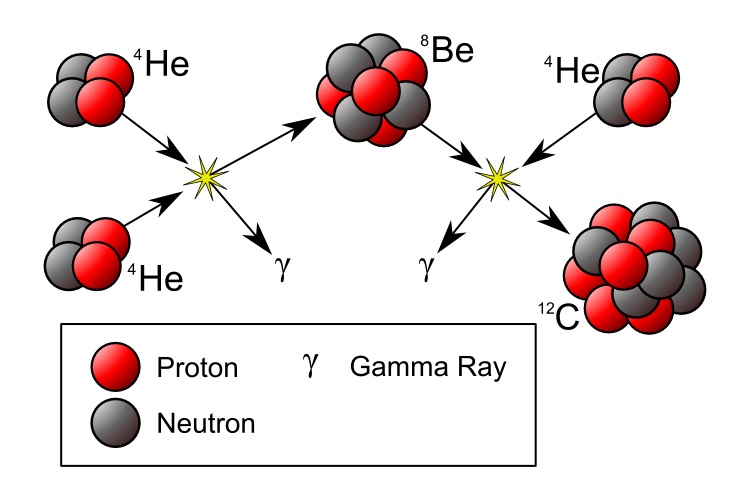
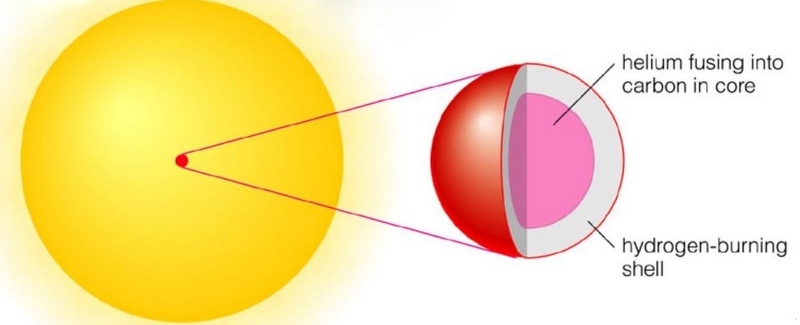
More massive stars will burn a lot faster to counteract gravity. The first stars may have had masses a few hundred times the solar mass, finishing the hydrogen it its core in only a few million years. What next? The core will contract and the temperature will increase. You might expect that fusion would start of two alpha particles into Beryllium (4 protons and 4 neutrons). But there is a problem, that Be isotope is not stable , it has a half live of only 8×10−17 s and decays back into two alpha particles. What will happen occasionally is that during its short lifetime, another alpha particle will collide and form Carbon (6p and 6 n). This is called the triple-alpha process and I will give more details in a separate appendix.
When this helium burning starts in the core, hydrogen burning will still continue in a shell around the core.
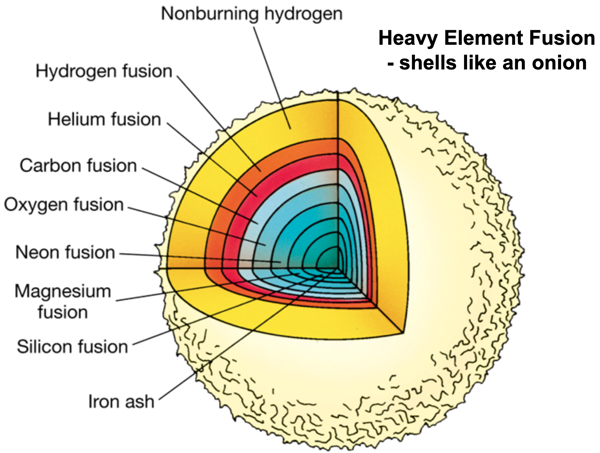
In the next phase, when the carbon core has been formed, carbon nuclei will fuse with alpha particles into Oxygen (8p and 8n) surrounded by a helium burning shell. And so on, Neon, Magnesium, Silicon etc. These fusion processes generate less energy than the hydrogen fusion and when iron is reached the fusion stops, fusion to heavier elements would cost energy! The star looks like an onion with its skins.
When there is no more energy to counteract gravity, the star will die in a spectacular fashion, releasing so much energy that for a short time it can be brighter than a whole galaxy. It is called a supernova. A large part of its mass will be ejected into the surrounding space and in the cataclysmic explosion many of the elements heaver than iron are formed. What remains of the star is a neutron star or a black hole.
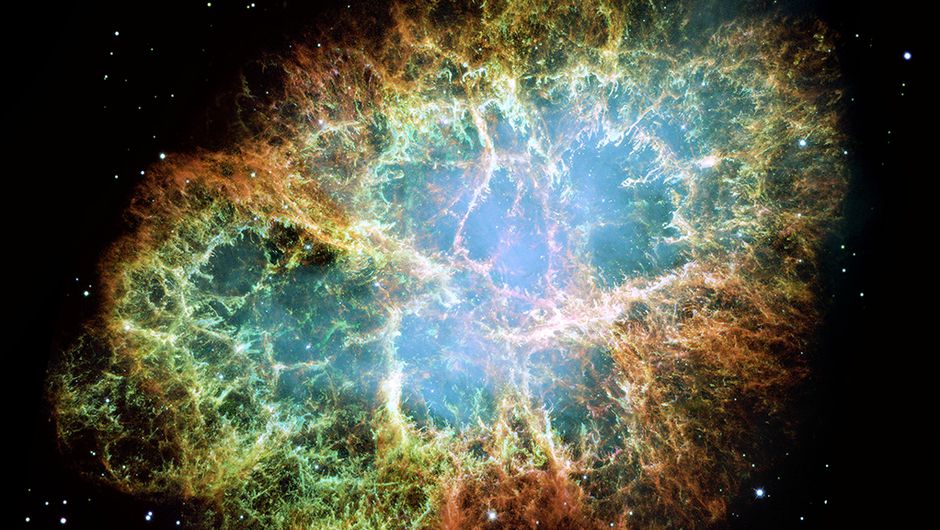
Recently I have written a blog about the Witch’s Broom nebula, the remnants of a supernova explosion. More details in that blog. The most famous of these supernova remnants is the Crab Nebula. The supernova has been recorded by Chinese astronomers in 1054. The center of the nebula contains a neutron star.
Here are a few more examples. They are all false-color images (see my Witch’s Broom blog). Here is a List of Supernova Remnants.
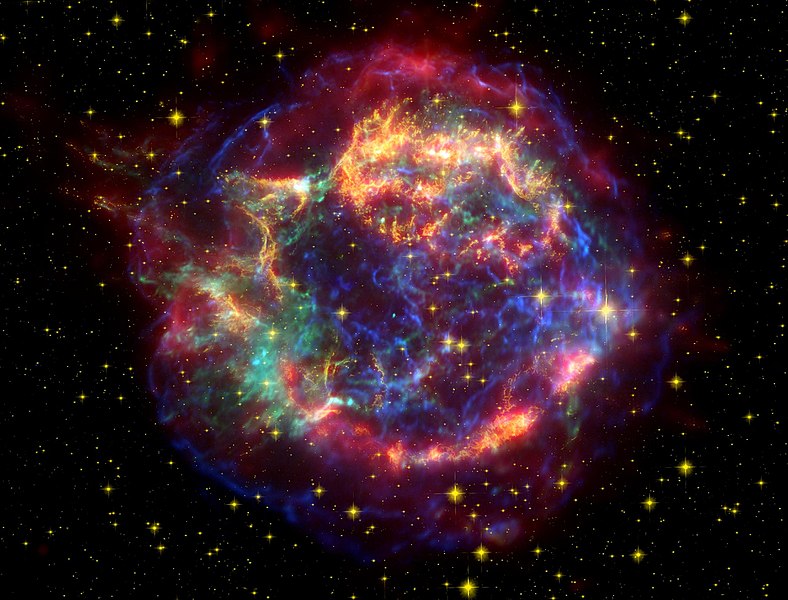
Cassiopeia A 
E0102 
G299 
W49B
As a result of these supernova explosions the clouds of interstellar gas became more and more “polluted”, no longer consisting of only hydrogen and helium. . It is from these clouds that new stars are born. For example our Sun, 4.9 billion year ago. Still mostly hydrogen and helium but about 0.1 % of the other elements. The big gas planets are also mostly H and He, but the rocky inner planets (Mercury, Venus, Earth and Mars) consist mainly of this 0,1 % other elements, as the hydrogen and helium have been “blown” away by the Sun.
Here is Earth, our Blue Marble, the iconic picture was taken by the crew of the Apollo 17 in 1972. Basically all its atoms have been forged in the interior of stars.
And the same holds for all living creatures, including us. Life on Earth is carbon-based, and each carbon atom has been fused in the interior of a star through the triple-alpha process.
We are Star Children
———————————————–
APPENDIX
The energy that is released when particles are fused is called the binding energy of that particle. For nuclear processes this energy is usually given in MeV (Million electronVolt). 1 MeV = 1.6×10-13 Joule. An alpha particle has a binding energy is 28.3 MeV. It is this (large) energy which generates pressure to counteract gravity in the interior of stars.
To fuse two alpha particles into beryllium is a different story. You have to add energy (it has a negative binding energy). Not much, 0.092 MeV, but as a result it is unstable, it will decay in two alpha particles. When the British astronomer Fred Hoyle in the 1950s studied the process how elements were formed in the interior of stars, he and others discovered this bottleneck.
During its short lifetime beryllium may fuse with another alpha particle into carbon and that will release energy, 7.367 MeV. The fiery furnace in the core of the star where fusion occurs would contribute another 0.3 MeV. Hoyle calculated that in most cases this “excited” carbon nucleus will decay into alpha particles instead of releasing the extra energy as gamma rays and settling down in its ground state. It could not explain the large amount of carbon found iin the universe.
UNLESS the carbon nucleus would have a so called resonance at an energy of ~7.7 MeV, Think about a soprano who can break a glass by letting it resonate with the frequency of her singing! But at that time no such resonance in the carbon nucleus was known.
Hoyle convinced his friend and colleague William Fowler, an experimental nuclear physicist, to search for such a resonance . And they found this excited level exactly at the energy predicted by Hoyle. This resonance level is now called the Hoyle state,
Was this a coincidence? Without this resonance level, carbon would not have been formed and carbon-based life would have been impossible.
Do we live in a Fine-tuned Universe ? Is the universe custom-made for “us” , the Anthropic Principle . Food for thought 😉


You really know how to blow peoples mind Jan, here in the UK its one in the morning and prior to going to bed, having read you missive, I think I will have a restless night mulling over it.
Was was there before the Big Bang ? How does the String Theory explain the existence of the universe ?