|
| 6. The Human Body |
|
 Adam and Eve by Cranach, 1533 |
To the left, Adam and Eve,as painted by Lucas Cranach the Elder in 1533. Until present times, conservative Christians criticise paintings like this one, because Adam and Eve should not have navels!
The image at the right shows a part of the famouse plaque which has been sent into space in 1971 with the Pioneer 10 spacecraft. It was designed by Carl Sagan and, fearing American puritanism, he simplified the female anatomy a bit, so aliens who might ever find this plaque in the future, will wonder how human reproduction took place :-).
The Pioneer 10 has left the Solar System by now and is on its way to the bright star Aldebaran, where it will arrive in about 2 million years.
|  Hello, we come from Earth |
We have seen in the preceding pages that the Universe is big, with hundreds of billions of galaxies, each having hundreds of billions of stars. The human body is very small compared with the universe.
But it is no problem to find huge numbers in the human body as well.
Like the Universe consist of galaxies, the Human Body consists of cells, the universal building blocks of life. How many cells are there in a human body?
Not easy to answer that question, human cells come in various sizes. But a rough estimate gives a number in the order of 50.000 billion. And that is roughly a factor 100 more than there are galaxies in the Universe!
Interested in another large number? What about the bacteria living inside us? Many of these unicellular organisms cause diseases, but others are essential for us to survive, like the bacteria in our gut who help us to digest our food. They outnumber our own cells with about a factor ten! Science journalist Robert Krulwich once remarked: "a visitor from outer space might think the human race is just one big chain of microbe hotels."
|
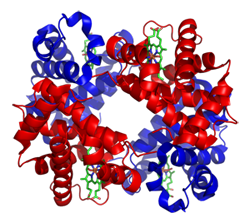 Structure model of Hemoglobin |
Each cell in our body contains molecules. How many? Again not an easy question. Of course a lot of simple water molecules H2O. But also many complicated macromolecules, culminating in the double helix of DNA, the carrier of our hereditary information. So let us not look at the molecules but at the atoms. How many atoms in an average cell?
Again a huge number, don't be surprised, in the order of 100.000 billion in each cell of our body, considerably more than there are stars in a galaxy.
The total number of atoms in our body depends of course upon size and weight, but a reasonable estimate is ~ 7×1027
What is be the distribution of this huge number over the different elements like hydrogen, oxygen, carbon etc? |
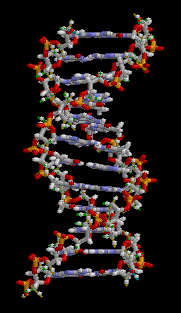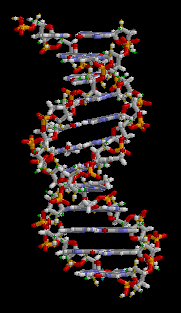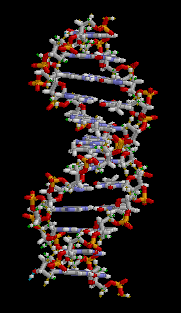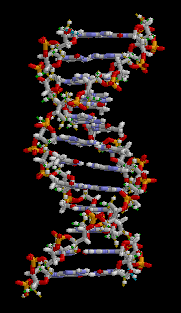 Structure of DNA |
As an example we give here the composition of the four nucleobases that form the alphabet for our genetic code. Guanine consists of 5 Carbon atoms (grey), 5 Hydrogen atoms (white), 5 Nitrogen atoms (blue) and 1 Oxygen atom (red). Adenine has a similar structure, but without the Oxygen atom, and so on.
Proteins like hemoglobin have a much more complicated structure, but also contain mainly H, C, N and O atoms. (The coloring in the structure model is different. Blue and red are proteins while the green part contains a Fe atom and is responsible for the oxygen carrying capability of the hemoglobin)
|
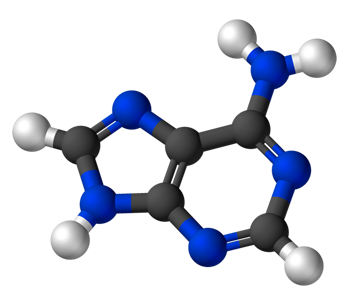 Adenine (C5H5N5) |
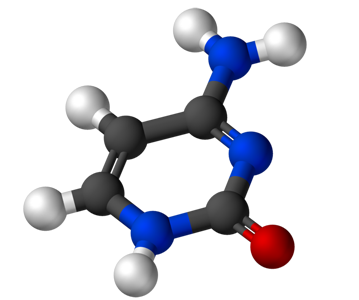 Cytosine (C4H5N3O) |
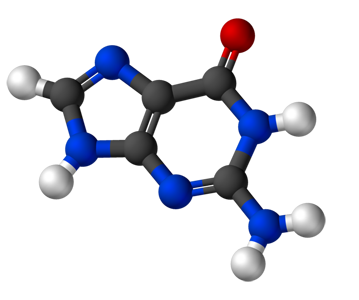 Guanine (C5H5N5O) |
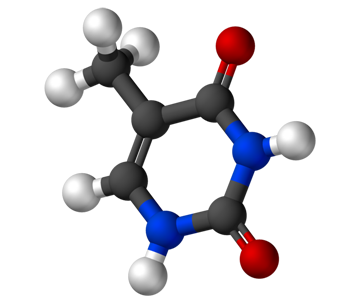 Thymine (C5H6N2O2) |
We can also determine the distribution of elements in a the human body as a whole. The table shows the approximate composition of a human body of 70 kg. It may come as a surprise that more than 50 kg consists of gaseous elements, oxygen, hydrogen and nitrogen, until you realise that much of the oxygen and hydrogen is bound in the form of water, the main component of humans (and all other life forms)!
|
Element | Mass | Element | Mass | Element | Mass |
Oxygen | 43 kg | Phosphorus | 780 g | Magnesium | 19 g |
Carbon | 16 kg | Potassium | 140 g | Iron | 4.2 g |
Hydrogen | 7 kg | Sulfur | 140 g | Fluorine | 2.6 g |
Nitrogen | 1.8 kg | Sodium | 100 g | Zinc | 2.3 g |
Calcium | 1.0 kg | Chlorine | 95 g | Silicon | 1.0 g |
|
We have limited the table content to elements which contribute at least 1 g. The list is much longer. All the elements mentioned in the table are essential for life. The first element, present in our body, but maybe not essential is Rubidium (0.68 g). Some elements in even smaller quantities we still need, like Cobalt (0.003 g) as part of Vitamin B12.
We can now formulate an important question: were all these elements formed when the Universe came into being, during the Big Bang?
The answer is a resounding NO! With the exception of hydrogen, all these elements have been formed millions or even billions of years later.
|
When? Where? How? Before we can answer these questions, we first must go one level deeper than the atoms. Because we know now that, even although "atomos" means indivisible, atoms consist themselves of even more fundamental paricles. |
|
|